Modern Periodic Trends
Ionization Energy
The minimum energy required to remove the electron from the outer most shell of gaseous atom that energy is called ionization energy
Defining first ionization energy
Definition
The first ionization energy is the energy required to remove the most loosely held electron from the outer most shell of an atom
This is more easily seen in symbol terms.

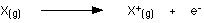
Here we go this is an atom you can see electron are revolving around a shell ,The energy is required to remove the first electron in the outer most shell it is called first ionization energy
and if we remove the second electron from the same shell it is called second ionization energy ,remember second ionization energy is always very high because strong nuclear force hold the electron so high energy is needed
Factor Effecting On Ionization energy
Nuclear charge
Atomic Size
Shielding effect
Number of shell
For Further Detail Click On This Link http://entertainmentme.blogspot.com/2014/03/factor-affecting-on-ionization-energy.html
Take care buddies
Published by Adil khan (mak)
Ionization Energy
The minimum energy required to remove the electron from the outer most shell of gaseous atom that energy is called ionization energy
Defining first ionization energy
Definition
The first ionization energy is the energy required to remove the most loosely held electron from the outer most shell of an atom
This is more easily seen in symbol terms.
Here we go this is an atom you can see electron are revolving around a shell ,The energy is required to remove the first electron in the outer most shell it is called first ionization energy
and if we remove the second electron from the same shell it is called second ionization energy ,remember second ionization energy is always very high because strong nuclear force hold the electron so high energy is needed
Factor Effecting On Ionization energy
Nuclear charge
Atomic Size
Shielding effect
Number of shell
For Further Detail Click On This Link http://entertainmentme.blogspot.com/2014/03/factor-affecting-on-ionization-energy.html
Take care buddies
Published by Adil khan (mak)
.jpg)

Post a Comment